For whoever asked for mole calculations. :)
It's detailed and has some example questions with working out, and some practice questions and tables for you to do too. Hope it helps!
Note that these are taken from my class book. They are not my materials.
It's detailed and has some example questions with working out, and some practice questions and tables for you to do too. Hope it helps!
Note that these are taken from my class book. They are not my materials.
1.15 calculate relative formula masses (Mr) from relative atomic masses (Ar)
Relative formula mass: the sum of masses of all the atoms in a formula
Relative atomic mass: the average mass of all the isotopes of an element
Examples:
Species | Formula | No. of atoms of each element in one molecule | Ar of each element | Sum of Ar | Relative Formula Mass (Mr) |
Carbon dioxide | CO2 | 1 carbon 2 oxygen | C=12 O=16 | 12 + (16 x 2) | 44 |
Oxygen | O2 | 2 oxygen | O=16 | 16 x 2 | 32 |
Aluminium oxide | Al2O3 | 2 aluminium 3 oxygen | Al=27 O=16 | (27 x 2) + (16 x 3) | 102 |
Aluminium sulphate | Al2(SO4)3 | 2 aluminium 3 sulphur 12 oxygen | Al=27 S=32 O=16 | (27 x 2) + (32 x 3) + (16 x 12) | 342 |
To calculate relative atomic mass (Ar): the sum of the relative abundance of each isotope multiplied by its mass number, divided by 100.
E.g. About 75% of all chlorine atoms have a mass number of 35 (18 neutrons), about 25% have a mass number of 37 (20 neutrons).
The relative atomic mass of chlorine is:
(75 x 35) + (25 x 37) / 100 = 35.5
1.18 carry out mole calculations using relative atomic mass (Ar) and relative formula mass (Mr)
n=m/Mr
Where n=number of moles, m=mass in grams and Mr=relative formula mass

e.g. how many moles are there in 120g of NaOH(s)?
n=m/Mr = 120g/40 = 3
There are 3 moles.
Try filling this out:
Substance | Formula | Mr | Mass of sample (g) | Number of moles in sample (n=m/Mr) |
E.g. Water | H2O | 18 | 9 | 0.5 |
Carbon dioxide | 88 | |||
Ammonia | 1.7 | |||
Sulphur dioxide | 0.64 | |||
Sulphur trioxide | 80 | |||
Hydrogen bromide | 24.3 | |||
Sulphuric acid | 0.098 | |||
Nitric acid | 3.15 | |||
Sodium nitrate | 21.25 | |||
Sodium carbonate | 53 |
Example:
What mass of hydrogen is produced when 192g of magnesium is reacted with hydrochloric acid?
Step 1: write a balanced equation for the reaction:
Mg + 2HCl à MgCl2 + H2
Step 2: under the equation, fill in all the information you have been given, and also the Mr of the species involved:
Equation | Mg + 2HCl à MgCl2 + H2 | |||
Mass | 192g | This is the value you want to find | ||
Mr | 24 | 2 | ||
n | ||||
Step 3: work out the number of moles of the reactant, using n=m/Mr then use the mole ratio of the equation to fill in the number of moles of the product:
Equation | Mg + 2HCl à MgCl2 + H2 | |||
Mass | 192g | This is the value you want to find | ||
Mr | 24 | 2 | ||
n | 8 | à 1:1 mole ratio | 8 | |
Step 4: now you have the Mr and number of moles of the product, rearrange n=m/Mr to find the mass:
m=n x Mr
Equation | Mg + 2HCl à MgCl2 + H2 | |||
Mass | 192g | 16g | ||
Mr | 24 | 2 | ||
n | 8 | à 1:1 mole ratio | 8 | |
Once you are familiar with the process, you don't need a calculation frame, but keep your workings well ordered and logical.
- What mass of oxygen is needed to react with 8.5g of hydrogen sulphide (H2S)?
2H2S + 3O2 à 2SO2 + 2H2O
Find out how many moles of hydrogen sulphide there are, since you have its mass.
n=m/Mr
n=8.5/34=0.25
Mole ratio= 2:3 (2 moles of H2S : 3 moles of O2)
(0.25/2) x 3 = 0.375 --- 0.375 moles of O2 react with 0.25 moles of H2S, now you want to find the mass.
n=m/Mr
m= n x Mr
m= 0.375 x 32 = 12g
- Railway lines are welded together by the thermite reaction, which produces molten iron. What mass of iron is formed from 1kg of iron oxide?
Fe2O3 + 2Al à 2Fe + Al2O3
n=m/Mr
n=1000g/160=6.25
Mole ratio= 1:2
6.25 x 2=12.5
m=n x Mr
m= 12.5 x 56 = 700g
Practice:
- What mass of sodium hydroxide is formed when 46g of sodium reacts with excess water?
- Calculate the mass of water formed when 32g of oxygen reacts with excess hydrogen.
- Calcium carbonate thermally decomposes to form calcium oxide and carbon dioxide. If 44g of carbon dioxide are collected, what mass of calcium oxide is formed?
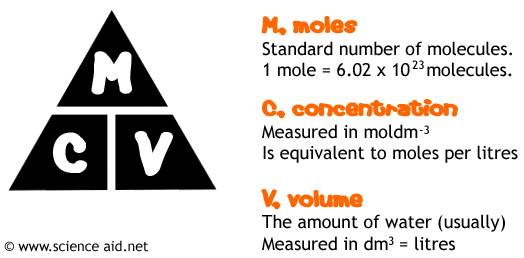
1.26 carry out mole calculations using volumes and molar concentrations
A solution contains a dissolved solute in a certain amount of solvent.
The concentration of a solution tells us how many moles of the solute are dissolved in one litre
(1 dm3) of the solvent.
(1 dm3) of the solvent.
The units for concentration are mol/dm3, and this is often shortened to M.
Concentration can also be measured in grams per litre.
n= v x c
Where v= volume (in dm3) and c= concentration (in mol/dm3)
NB: sometimes they give you the volume in cm3 so be careful, convert it to dm3 by dividing by 1000
(1 dm3 = 1000 cm3)
Or, you can just use: n= (v x c) / 1000
Remember to convert cm3 to dm3!
Solution | Formula | Concentration (mol m-3) | Concentration (g dm-3) | Volume | Number of moles |
Sodium hydroxide | NaOH | 1 | 500 cm3 | ||
Hydrochloric acid | HCl | 0.5 | 2 dm3 | ||
Sodium chloride | NaCl | 58.5 | 4 | ||
Potassium chloride | KCl | 200 cm3 | 0.2 | ||
Ammonium chloride | NH4Cl | 0.25 | 250 cm3 | ||
Silver nitrate | AgNO3 | 2 | 0.5 | ||
Lithium iodide | LiI | ||||
Sulphuric acid | H2SO4 | 0.2 | 1.8 | ||
Potassium nitrate | KNO3 | 150 cm3 | 0.15 |
- 25cm3 of a solution of 0.1M NaOH is neutralised by 50cm3 of HCl. What is the concentration of the HCl?
NaOH + HCl à NaCl + H2O
NB: remember to convert volumes to dm3!
n= v x c
n= 0.025 x 0.1=0.0025 mole
Mole ratio= 1:1, so moles of HCl= 0.0025 mole
C= n/v
C= 0.0025/ 0.05=0.05 mol/dm3
Practice:
- What mass of silver chloride precipitate will be produced if 25 cm3 of 0.1M silver nitrate is added to excess sodium chloride solution?(Answer may not be exact, so you give it to 2 significant figures here because the most exact data they give you here is to 2 sig. fig. which is the volume.)
- What mass of magnesium will react with 10cm3 of 1M HCl? Extra: and what volume of hydrogen will be formed? (1 mole of any gas at RTP is 24dm3)
Answers:
Substance | Formula | Mr | Mass of sample (g) | Number of moles in sample (n=m/Mr) |
E.g. Water | H2O | 18 | 9 | 0.5 |
Carbon dioxide | CO2 | 44 | 88 | 2 |
Ammonia | NH3 | 17 | 1.7 | 0.1 |
Sulphur dioxide | SO2 | 64 | 0.64 | 0.01 |
Sulphur trioxide | SO3 | 80 | 80 | 1 |
Hydrogen bromide | HBr2 | 81 | 24.3 | 0.22 |
Sulphuric acid | H2SO4 | 98 | 0.098 | 0.001 |
Nitric acid | HNO3 | 63 | 3.15 | 0.05 |
Sodium nitrate | NaNO3 | 85 | 21.25 | 0.25 |
Sodium carbonate | Na2CO3 | 106 | 53 | 0.5 |
2 Na | + | 2 H2O | à | 2 NaOH | + | H2 |
46g | ? | |||||
1:1 ratio | ||||||
23g | : | 40g | ||||
46g | : | 80g | ||||
2. Calculate the mass of water formed when 32g of oxygen reacts with excess hydrogen.
2 H2 | + | O2 | à | 2 H2O |
1 | : | 2 | ||
32g | : | 36g (m = n x Mr m = 2 x 18) |
3. Calcium carbonate thermally decomposes to form calcium oxide and carbon dioxide. If 44g of carbon dioxide are collected, what mass of calcium oxide is formed?
CaCO3 | à | CaO | + | CO2 |
1 | : | 1 | ||
56g | : | 44g | ||
*Remember that one mole of a substance is equivalent to its RFM in grams. For instance with CaO, the Mr of Ca is 40, and for O it is 16. Thus the RFM of CaO is 40 + 16 = 56. And since 44g of CO2 is 1 mole, then one mole of CaO must have been produced too (look at balanced equation), and 1 mole of CaO = 56g. | ||||
Solution | Formula | Concentration (mol m-3) | Concentration (g dm-3) | Volume | Number of moles |
Sodium hydroxide | NaOH | 1 | 40 | 500 cm3 | 0.5 |
Hydrochloric acid | HCl | 0.5 | 18.25 | 2 dm3 | 1 |
Sodium chloride | NaCl | 1 | 58.5 | 4 dm3 | 4 |
Potassium chloride | KCl | 1 | 74.5 | 200 cm3 | 0.2 |
Ammonium chloride | NH4Cl | 0.25 | 13.375 | 250 cm3 | 0.0625 |
Silver nitrate | AgNO3 | 2 | 340 | 0.25 dm3 | 0.5 |
Lithium iodide | LiI | 1 | 134 | 1 dm3 | 1 |
Sulphuric acid | H2SO4 | 0.2 | 19.6 | 9 dm3 | 1.8 |
Potassium nitrate | KNO3 | 1 | 101 | 150 cm3 | 0.15 |
What mass of silver chloride precipitate will be produced if 25 cm3 of 0.1M silver nitrate is added to excess sodium chloride solution?(Answer may not be exact, so you give it to 2 significant figures here because the most exact data they give you here is to 2 sig. fig. which is the volume.)
AgNO3 | + | NaCl | à | AgCl | + | NaNO3 |
1 | : | 1 | ||||
Moles of AgNO3 | = | v | x | C | ||
= | 0.025 | x | 0.1 | |||
= | 0.0025 mol | |||||
Moles of AgCl | = | 0.0025 mol | Due to 1:1 ratio | |||
m | = | n | x | RFM | ||
= | 0.0025 | X | 143.5 | |||
≈ | 0.36g | (volume is only 2 sig. fig.) | ||||
2. What mass of magnesium will react with 10cm3 of 1M HCl? Extra: and what volume of hydrogen will be formed? Assume RTP conditions -- 25°C and 1 atm (1 mole of any gas at RTP is 24dm3)
Mg | + | 2 HCl | à | MgCl2 | + | H2 |
1 | : | 2 | : | 1 | ||
Moles of HCl | = | v | x | C | ||
= | 0.01 | x | 1 | |||
= | 0.01 moles | |||||
Moles of Mg | = | 0.005 | (due to 1:2 ratio, 0.01 moles of HCl will react with 0.005 moles of Mg) | |||
Mass of Mg | = | n | x | RFM | ||
= | 0.005 | x | 24 | |||
= | 0.012g | |||||
1 mole of H2 | = | 24 dm3 | = | 24,000 cm3 | ||
0.005 mole of H2 | = | 0.005 | x | 24,000 | ||
= | 120 cm3 | |||||
So that's done.. hope the answers help. And good luck with your IGCSEs! I really can't update like this anymore, apologies but this was just to complete this post. Hope it helps anyway. :)
Tidak ada komentar:
Posting Komentar